Scientific Motivation:
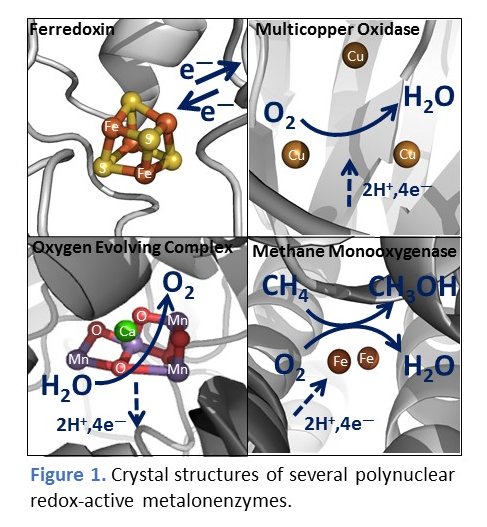
Redox active transition metal ions are ubiquitous elements of life and play crucial roles in catalysis of many difficult biochemical transformations
such as water oxidation (photosynthesis), nitrogen reduction to bioavailable ammonia (N2 fixation), CO2 reduction to CH4 and methane oxidation to
methanol etc. All these systems require radicals to function. Even more, it appears that radical enzymes dominate bioinorganic chemistry.
Catalytic pathways of such enzymatic reactions have been evolutionarily tuned over many million years and are therefore extremely effective
and inspiring for both synthetic chemistry and biology in the development of new green catalysts and potential drugs. Despite the tremendous
progress achieved in bioinorganic chemistry during the two last decades, goals in the field (eg. unraveling reaction mechanisms and designing
biomimetic complexes with novel functions) remain very challenging due to complex geometric and electronic structures, a facet which requires
scientific approaches across multiple disciplines such as bioinorganic chemistry, synthetic chemistry, spectroscopy, electrochemistry,
and theoretical (quantum) chemistry. This is particularly true for chemistry involving catalysts with more than one redox-active transition
metal center (ie. polynuclear transition metal complexes).
In our lab, we focus mainly on the following topics:
Redox Properties and Reactivities of Mono and Binuclear Nonheme Iron Active Sites
Figure from Srnec et al JACS 2014
Adapted from Figure from Srnec et al JBIC 2016
Radical Catalysis By Enzymatic and Biomimetic Polynuclear Transition-Metal Active Sites
SAM radical intermediates
Electronic Structure Contributions to Enzymatic Selectivity
Figure from Srnec & Solomon JACS 2017
Figure from Srnec et al Dalton Transaction 2014
Theoretical Bioinorganic Spectroscopy
Figure adapted from Srnec et al PNAS 2012
Computational Electrochemistry
Figure from Srnec et al JPCLett 2016